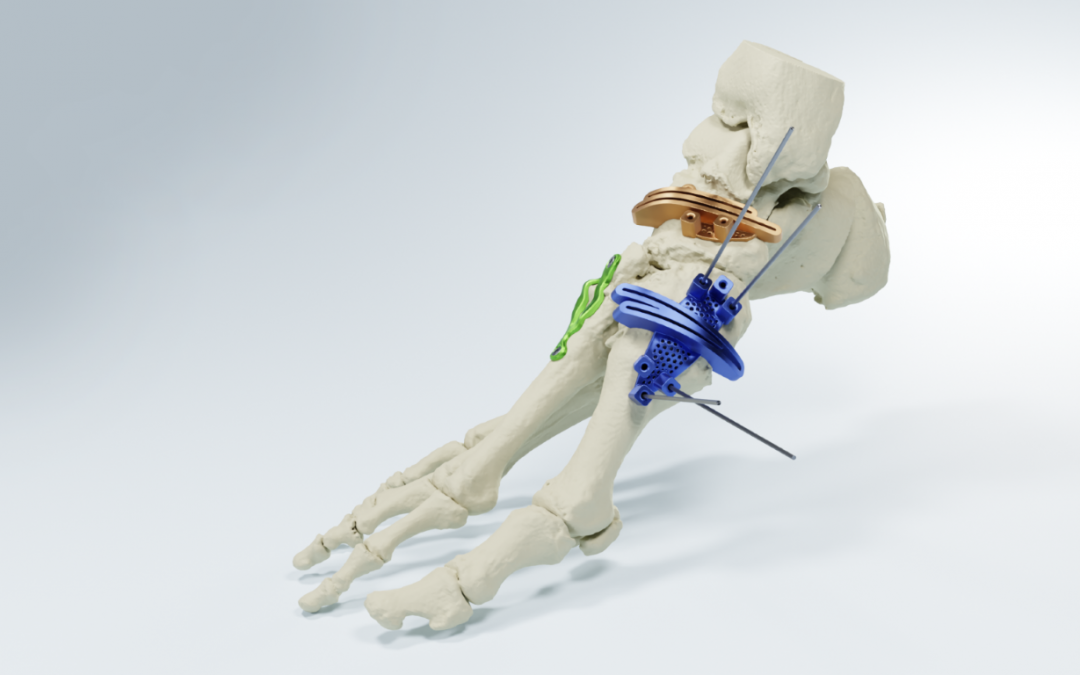
Dallas-based MedCAD has announced the 510(k) clearance of its AccuStride® Foot and Ankle System, making these patient-specific precision devices available to surgeons. The unique design of these devices, coupled with the company’s proprietary software, will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.