News

MedCAD Launches Personalized Case Tracking Web App “MyMedCAD”
MyMedCAD – a personalized case tracking web app designed to provide customers with increased visibility and control over the medical device customization process.
Online Service Request Moves to MyMedCAD
Release notes for Online Service Request
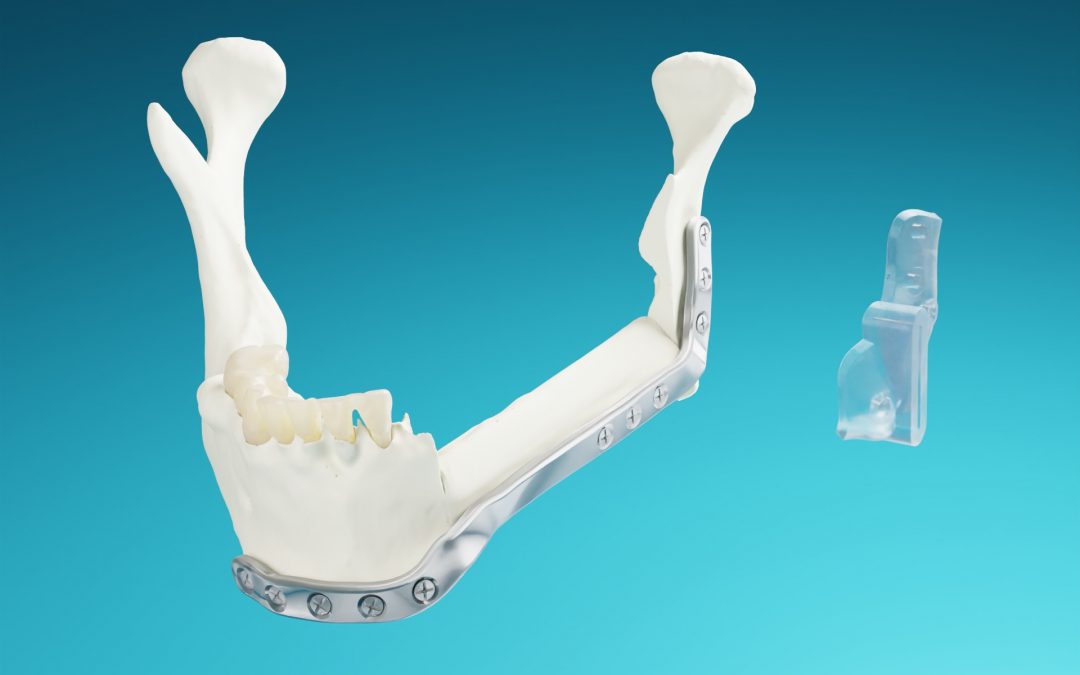
MedCAD Launches AccuPlate® Patient-Specific Plates after 510(k) Market Clearance
MedCAD is proud to officially announce FDA 510(k) market clearance for AccuPlate® Patient-Specific Plates for mandibular reconstructive surgery.

MedCAD Launches New Website and Brand Refresh
The culmination of years of research and development, long hours, a relentless obsession with customer service and new market clearances has earned MedCAD a brand refresh that drives forward our mission of Restoring Humanity One Patient at a Time.

MedCAD Receives 510(k) Market Clearance for New AccuPlan® System
MedCAD is proud to announce 510(k) market clearance for its new AccuPlan System through the Food and Drug Administration (FDA).

CPT Codes for Surgical Planning, Guides and 3D Models
Physicians and suppliers are excited because as of July 1, 2019, the American Medical Association (AMA) is using provisional CPT® Codes corresponding to Surgical Planning, Guides, 3D Anatomical Models, and corresponding products and services.
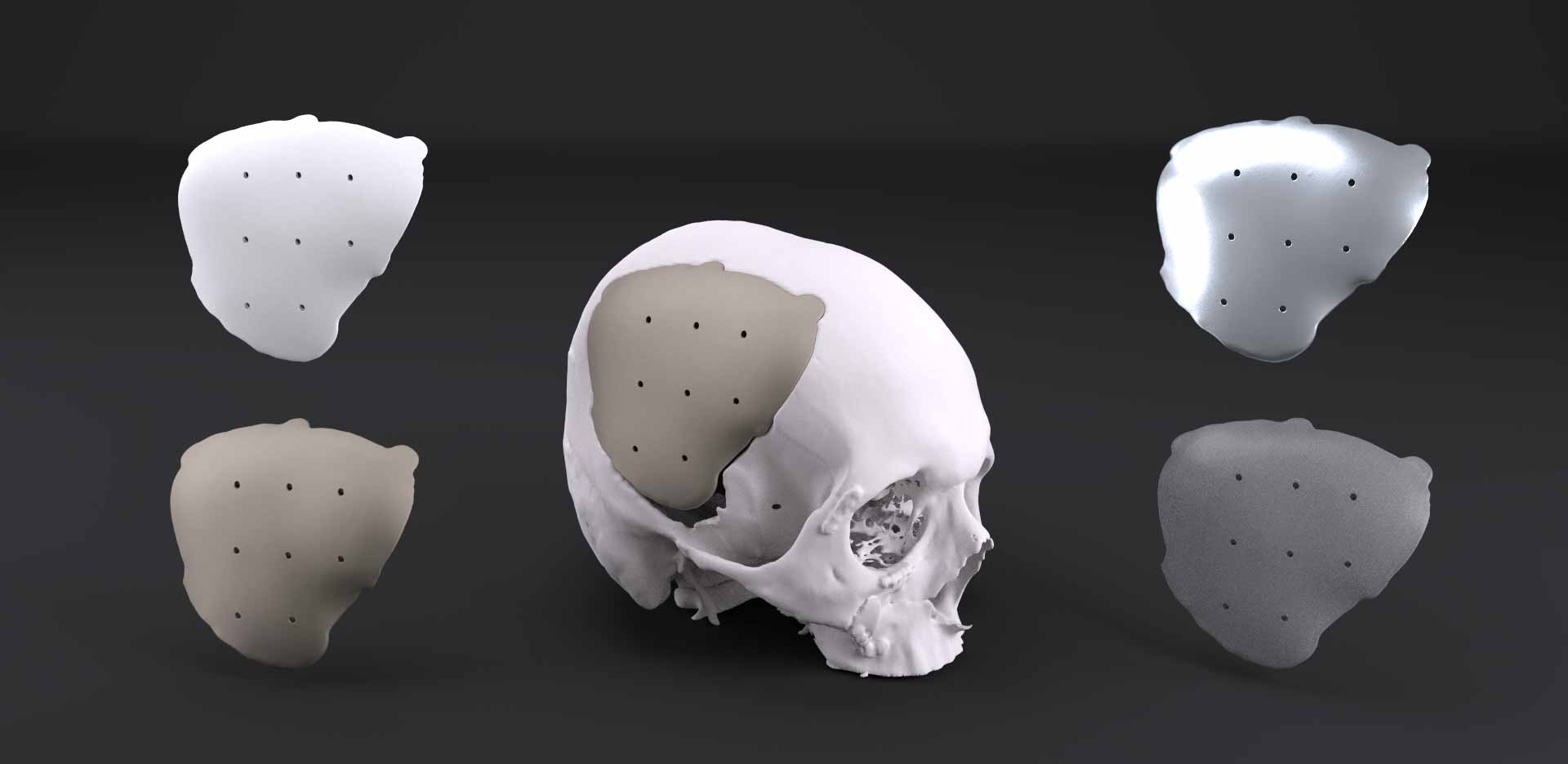
PEEK vs PEKK, PMMA, Porous Polyethylene and Titanium Mesh
When choosing cranioplasty implants, how do you know which option is best? Know the pro’s and con’s of each material and application.
