MyMedCAD – a personalized case tracking web app designed to provide customers with increased visibility and control over the medical device customization process.

News

MyMedCAD – a personalized case tracking web app designed to provide customers with increased visibility and control over the medical device customization process.
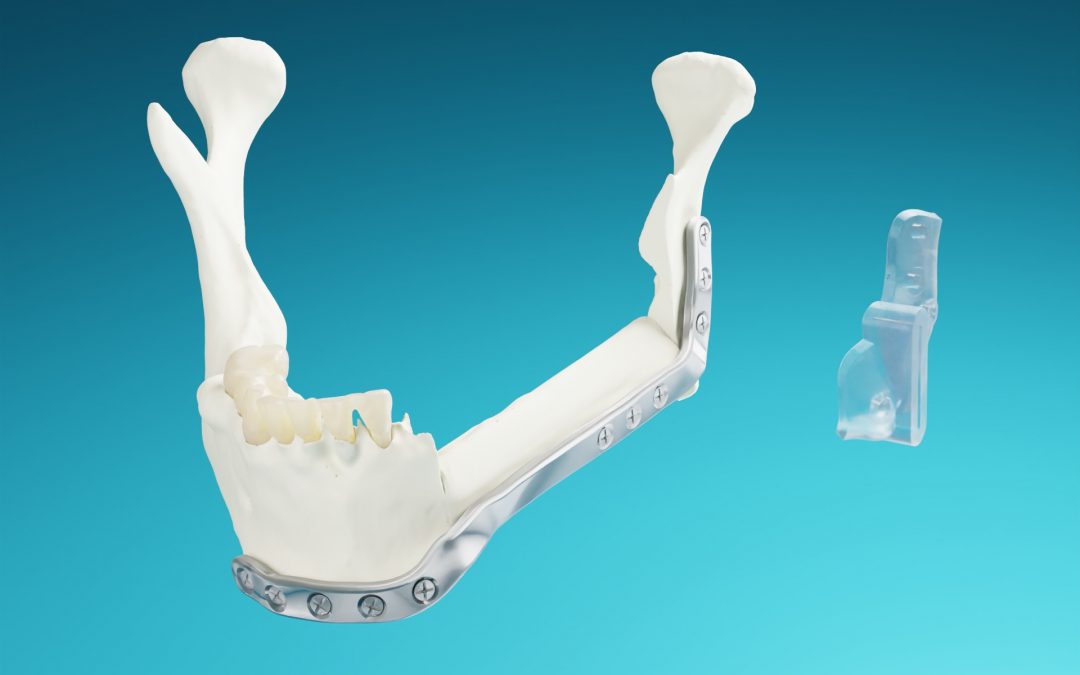
MedCAD is proud to officially announce FDA 510(k) market clearance for AccuPlate® Patient-Specific Plates for mandibular reconstructive surgery.

The culmination of years of research and development, long hours, a relentless obsession with customer service and new market clearances has earned MedCAD a brand refresh that drives forward our mission of Restoring Humanity One Patient at a Time.

MedCAD is proud to announce 510(k) market clearance for its new AccuPlan System through the Food and Drug Administration (FDA).
Last December, the first AccuShape cranial implant was used to treat the stroke patient in Florida. The patient had a serious skull defect and required a cranioplasty. The patient-specific AccuShape PEEK plate was the first produced by MedCAD since the company received their 510k FDA approval in October.